Wtf is Fire? 🔥
Morning Pyromaniacs!

Have you ever just… looked into fire?

Deep into the flames.

And thought… what even is this stuff?
Flames are obviously not a solid, nor a liquid.
But it also technically can’t be a gas because fire can burn out, a gas can’t.
Ok, the nerds among you might say, “but what about the 4th state of matter, plasma?”

The thing is, this is also wrong.
Plasma only exists when gas is exposed to an electric field or SUPER hot temperatures, like 10,000 degrees.
(And fire only burns at a few hundred degrees)
So if fire isn’t a solid, liquid, gas, or plasma, what actually is it?
Well, it turns out fire isn't actually matter at all, it's actually just a chemical reaction that we can see, hear, and feel.
This reaction requires 3 things:

And when these 3 come together, combustion happens, releasing a ton of heat and hot gasses.

The flame is actually just fast moving gas particles, releasing energy as visible light.
Let's get into a little more detail…
When wood starts to burn, the walls of the cells in the wood decompose.
Releasing a bunch of sugars and other molecules into the air.

These molecules react with oxygen in the air.

Creating Carbon Dioxide and water.
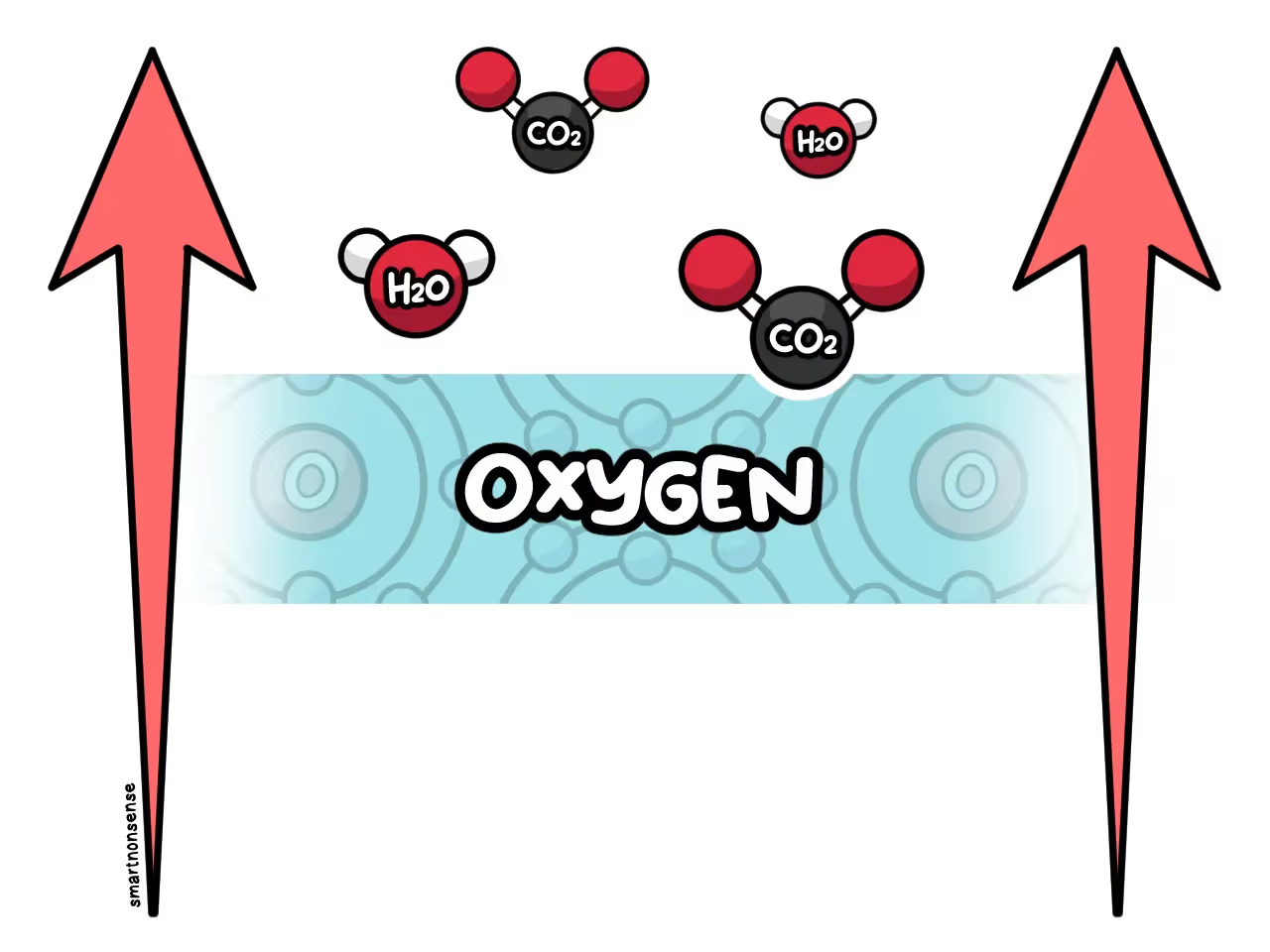
At the same time, any trapped water in the logs starts to vaporize and expand.
Breaking open the wood and eventually escaping, creating a satisfying crackle sound.

As the fire heats up more and more, the carbon dioxide and water vapor start to expand.
And as they get less dense, they rise into a column of smoke above the open flame.

Creating a beautiful… dancing array of light and smoke.

Stay Cute,
Reece, Henry & Dylan 🌈
If you’re that sexy friend, subscribe here.
Get smart about nonsense🌈
Join 100,000+ subscribers and get our daily comic explaining nerdy stuff like you’re 5.





